- Visibility 414 Views
- Downloads 134 Downloads
- DOI 10.18231/j.ijcaap.2021.002
-
CrossMark
- Citation
Toxicokinetics an essential tool in drug discovery: A review article
- Author Details:
-
Sandhya *
Introduction
Toxicokinetics refers to the study of absorption, distribution, metabolism/biotransformation, and excretion (ADME) of toxicants/xenobiotics in relation to time. Absorption describes the entrance of through the air, water, food, or soil. Once a chemical is inside a body, it can be distributed to other areas of the body through diffusion or other biological processes.[1]
In exposed organisms, metabolism is an important factor in determining the bioaccumulation, fate, pharmacokinetics (or toxicokinetics), and toxicity of contaminants.[2]
Contaminant exposure can results in the induction of Phase I cytochrome P450 monoxygenase (CYP) enzymes and Phase II conjugation enzymes (e.g- glucuronosyl transferase, sulfotransferases, and gluathione-S-transferases).[3]
Knowledge of toxicokinetics is for evaluating toxicity or for selecting dosages to be administered to animals in toxicology studies. A goal of most toxicity research is to infer expected human health risks from the results in test animals are commonly exposed to extremely high concentration of test chemicals, by unusual routes of administration, for their entire life time.[4] The challenge is to extrapolate from animal results to predict effects in human population that have very different exposure patterns.
Toxicokinetics involves the generation of kinetic data to assess systemic exposure, either as an integral component of preclinical toxicity studies, or in specially designed supportive studies.[1] These data help to understand the relationship between observed toxicity and administered dose. They also play a role in the clinical setting, assisting in the setting of plasma limits for early human exposure and in the calculation of safety margins.[5]
The primary objective of toxicokinetics is to describe the systemic exposure achieved in animals and its relationship to dose level and the time course of the toxicity study.[6] Toxicokinetics is largely reflective of the ADME of a molecule as it moves through the body of an organism. As with all animal studies, interspecies differences in ADME should be considered prior to clinical human testing and during data extrapolation.[7] If correctly utilized, Toxicokinetics studies can provide quantifiable endpoints to better explain negative effects that may arise during toxicity studies6.
Toxicokinetics/Pharmacokinetics
All drugs under development to treat human diseases must undergo a series of, Toxicokinetics/Pharmacokinetics investigations in animals and humans to gain an understanding of the their properties. [8]
Toxicokinetics are shares important parameters, like Cmax and AUC, with preclinical Pharmacokinetics, the studies are:
The primary goal of TK is to correlate findings of toxicity (not therapeutic efficacy) with a corresponding level of exposure to an experimental drug compound.
The TK arm of a nonclinical toxicology study generally has fewer time points, fewer subjects and fewer endpoints compared to nonclinical and clinical PK studies.
Half-life (t1/2) may not be accurately determined in TK studies (although it is frequently estimated) due to relatively sparse sampling of blood or plasma for concentration-time analysis. [9]
Toxicokinetics
Clinical veterinary toxicology
Pharmacokinetics and toxicokinetics are also important concepts regarding food residue and safety issues as well as drug withdrawal times in food-producing animal. [10] In 1982, the U.S. Department of Agriculture (USDA) Extension Service began involvement in an educational/service project to further enhance the safety of animal-derived foods. The Residue Avoidance Program, which was initially founded by the USDA Food Safety and Inspection Service (FSIS), targeted the area of xenobiotics residues. [11]
Models and methods for in vitro toxicity
Toxicokinetics study is essentially required to related the dose or chemical concentration and the mode of action of the chemical and its various metabolites. The toxicokinetic process is responsible for the distribution and formation of various chemical entities at the target tissue, which is further responsible for determining the dose at toxicological site. [12] The basic toxicokinetic parameter is based on in vitro and in silico studies, which detects the potential of accumulation and the potential of distribution or inhibition of chemical in the tissues/organs. [13]
In vitro approaches retrieve information of prime importance in the area of toxicokinetic studies. One of the representative model, the physiologically based toxicokinetic model, can be obtained by including the kinetics of metabolism by the liver and any other organ capable of biotransforming. [14]
Preclinical drug development
Toxicokinetics is the generation of pharmacokinetic data as a part of various toxicity studies in order to assess systemic exposure. [15] The measurement of peak and total exposure in these studies helps to determine the relationship between the toxicological effects and the exposure. The data on exposure is more relevant for comparing effects in animals and man. This is because the pharmacokinetics of a drug varies extensively between the species. [16]
These studies generally include repeated-dose toxicity studies, reproductive, and carcinogenicity studies. Since, single dose studies are usually done before a bio-analytical method has been developed, toxicokinetic monitoring cannot be integrated in these studies. [16]
Normally, in the case of large animals, blood samples for the generation of toxicokinetic data are collected from main study itself. However, in the case of smaller species satellite group may be required. ICH Guidance S34 provides detailed recommendation on toxicokinetic assessment. [17]
Toxicity and safety evaluation of pesticides
Toxicokinetics studies provide data on the ADME of the chemical pesticide. In general terms, how does it enter the body, where does it go, and what happens to its. These studies will also provide information on other parameter of interest, including difference between small and large doses, and single versus multiple exposures. [18]
Understanding the toxicokinetics of the pesticide may also enable more appropriate selection of doses and routes of administration used in many of the laboratory studies, as well as toxicokinetic consideration, will assist in determining the appropriate route of exposure and duration of study.
Toxicity and Safety Testing
Metabolism and Toxicokinetics
Toxicokinetics describes what an animal's body does with a drug or chemical or its metabolites following administration of the parent drug, typically at a high dose relative to the therapeutic on or effective dose. The 1994 ICH S34 guideline identifies toxicokinetics as an integral part of a nonclinical testing program because it supports species selection and the treatment regimen, enhances the toxicity data, and provides comparisons with clinical data, which is important in assessing risk and safety in humans. The guidance also notes the role for toxicokinetics in interpretation of similarities and differences across species, which may result from difference in protein binding, tissue uptake, receptor properties, and metabolic profiles. [19]
Toxicokinetics from in vitro studies contributes to understanding mechanisms of action and species differences. For example, Carney et al. (2008) used WEC to ethylene glycol (EG) is teratogenic in rats but not rabbits. Maximal levels of unchanged EG in rabbits were comparable to those reported for pregnant rats while maximal levels of the teratogenic metabolite, glycolic acid, in rabbit maternal blood and embryo were 46% and 10% of the respective levels in rats. [20]
Modeling Toxicity
Toxicokinetics Modeling
Toxicokinetic models integrate the uptake, metabolism, and excretion of chemicals in an individual organism. [20] The major aim of the modeling is to get the internal effective concentration (IEC), in the simplest case, the organism is considered as a single compartment, and both the uptake and the excretion of chemicals can be thought of as simple exponential uptake and loss. [21] Mathematical modeling of multi-compartment, The compartmental models require only limited time-series data for a compound in a reference compartment to allow estimation of pharmacokinetic parameters, the data are collected from different tissues/physiological compartments and their perfusion is take into account, the compartments are chosen so that they represent physiologically relevant entities. [22]
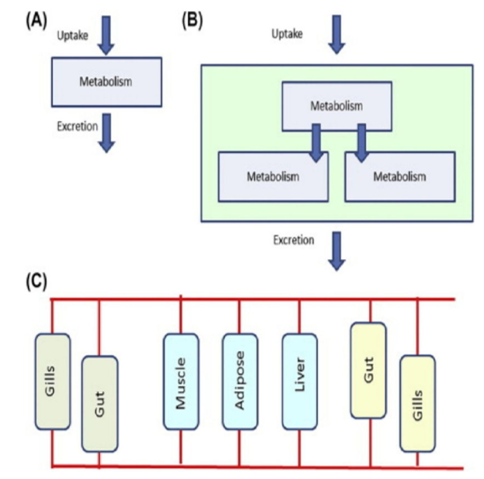
The single-compartment model
The multi-compartment model. In multi-compartment models, the calculation of uptake and excretion become complex. The different compartments are defined operationally and have no preconceived notions about physiological relationships.
Physiological based toxicokinetic (PBTK) models divide uptake, metabolism, and excretion (signified in the figure by different colors) into functionally relevent compartments. [23]
Orally delivered nanoparticle drug delivery systems for dental application and their toxicity on system organs
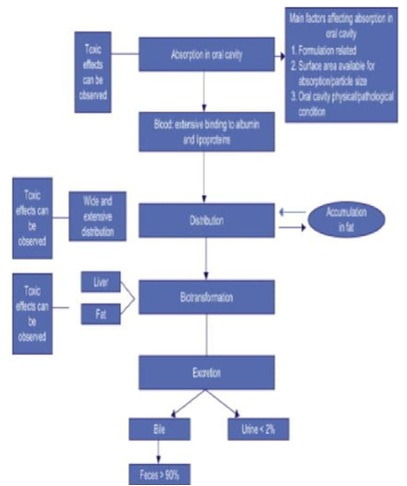
Principles of Toxicokinetics
Toxicokinetics is the mathematical description of the uptake and disposition of a chemical in the body. Toxicokinetics modeling is usually implemented by describing the time course of the amount or concentration of the parent substance and its metabolites in one or several body compartments.[24] Toxicokinetics models may also be used to strengthen experimental results by linking data obtained under different experimental conditions in uniform model. [25] One might, for example, use a single model to explain the toxicokinetics after different routes of administration or to describe uniformly the toxicokinetics of a family of related chemicals.[26] Toxicokinetics modeling may also be used as an aid in the development of biological exposure monitoring. [24]
Some may consider that pharmacokinetics is all to do with arithmetic and others that toxicology is only concerned with chemicals that make us sick. In fact, neither discipline can develop without the other, especially not understanding the mechanisms of toxic reaction.[27]
Toxicology Testing
Toxicology programs have evolved from a traditional exploration of chemistry and applied toxicity of chemicals and drugs to a more comprehensive study of toxicology and toxicology testing as independent entities. Toxicology testing starts with basic toxicological principles, including absorption, distribution, metabolism, and elimination of toxins, including chemical and drug.[28]
This introductory material is useful in understanding the applications of toxicology testing. The fundamental principle of toxicology testing in animals in greater detail. This section explains acute toxicity studies as well as subchronic and chronic studies performed on animal.[29]
Special emphasis is placed on study design and determination of classical indicators for acute and chronic testing, such as the LD50. [30]
Applications of Toxicokinetics
Toxicokinetics, toxicodynamics and toxicogenomics method are the potential tools in human health risk assessment.[31] Application of these techniques give detail knowledge about the drug kinetics and metabolism; improved assessment strategy with greater efficiency, use fewer animals and provide better data for risk assessment purpose; rescue at-risk programs in preclinical/early clinical development; proactively screen/evaluate leads at early stages using predictive tools for toxicity and mechanism of action, develop preclinical biomarkers of drug response and toxicity, adoption of toxicity management approaches to improve the therapeutic outcomes.[32]
Studies reveal the drug toxicity at molecular as well as genetic level that helps researchers and physicians to reduce the undesired effects of drug.TK/TD/TG approaches are very important to develop experiments designed to understand the molecular basis of drug toxicities.[31]
Toxicokinetics evaluation is important in drug development stages. This evaluation should constitute effective analytical methods having good accuracy and precision, adequate sampling, drug and metabolites evaluation both in animals and humans and sufficient results evaluation.[33]
Toxicokinetics data is important to know the toxic responses to that of drug in preclinical which can be used to set safe dose for clinical use of new drugs. It also gives support to mode of action analysis and extrapolation across exposure routes.[34] Toxicokinetics used in other areas of pharmacokinetics which act as a biomarkers for doing screening studies which provide data for allometric species scaling, measuring drug levels in non-plasma samples (tissues, urine and bile). Even though toxicokinetics evaluation is only a small part of the process of understanding the fate of a drug, it has a vital part in drug development.[35]
The evolution of toxicity whether as discrete or sequential, single or multiple events, each effects measured or observed can be identified by toxicokinetic data.[36]
The route of administration and the nature of the formulation to be used in humans, as well as the therapeutic indication for which the compound is intended can also be detected.[35]
Conclusion
Toxicokinetics studies is to describe the systemic exposure achieved in animals and its relationship to dose level and the time course of the toxicity study. Increased implementation of toxicokinetic sampling in all stages of toxicity testing could provide significant improvement in terms of efficiency, relevance, reliability, time constrains and budget.
Conflicts of Interest
All contributing authors declare no conflicts of interest.
Source of Funding
None.
References
- V K Batra, A Yacobi, A Yacobi, JP Skelly, VK Batra. An overview of toxicokinetics. Toxicokinetics and new drug development. Pergamon, 2015. [Google Scholar]
- H Boxenbaum. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokin Biopharm 2016. [Google Scholar]
- H Boxenbaum. Interspecies pharmacokinetic scaling and the evolutionarycomparativeparadigm. Drug Metab Rev 2014. [Google Scholar]
- D B Campbell, RMJ Ings. New approaches to the use of pharmacokinetics in toxicology and drug development. Human Toxicol 2018. [Google Scholar]
- J M Collins, D S Zaharko, R L Dedrick, B A Chabner. Potential roles for preclinical pharmacology in phase I clinical trials. Cancer Treat Rep 2016. [Google Scholar]
- Group. EORTC Pharmacokinetics and Metabolism Group , Pharmacokinetically guided dose escalation in phase I clinical trials. Commentary and proposed guidelines. Eur J Cancer Clin Oncol 2017. [Google Scholar]
- L Gianni, L Vigano, A Surbone, D Ballinari, P Casali, C Tarella. Pharmacology and clinical toxicity of 4′-iodo-4′-deoxydoxorubicin: an example of successful application of pharmacokinetics to dose escalation in phase I trials. J Natl Cancer Inst 2018. [Google Scholar]
- D A Graves, C S Locke, K T Muir, R P Miller. The influence of assay variability on pharmacokinetic parameter estimation. J Pharm Biopharm 2015. [Google Scholar]
- D R Hawkins, L F Chasseaud. Reasons for monitoring kinetics in safety evaluation studies. Arch Toxicol 2015. [Google Scholar]
- A M Monro. The role of metabolism studies in drug safety evaluation. Drug Dev Commun 2016. [Google Scholar]
- J Mordenti. Pharmacokinetic scaleup: accurate prediction of human pharmacokinetic profiles from animal data. J Pharm Sci 2015. [Google Scholar]
- R D Smyth, G H Hottendorf. Application of pharmacokinetics and biopharmaceutics in the design of toxicological studies. Toxicol Appl Pharmacol 2014. [Google Scholar]
- P G Welling, LZ Benet, N Massoud, JG Gambertoglio. Effects of gastrointestinal disease on drug absorption. Pharmacokinetic basis for drug treatment 2014. [Google Scholar]
- A Yacobi, B L Kamath, C M Lai. Pharmacokinetics in chronic animal toxicity studies. Drug Metab Rev 2016. [Google Scholar]
- A Sato, T Nakajima. A vial-equilibration method to evaluate the drug-metabolizing enzyme activity for volatile hydrocarbons. Toxicol Appl Pharmacol 2015. [Google Scholar]
- G W Jepson, D K Hoover, R K Black, J D Mccafferty, D A Mahle, J M Gearhart. A partition coefficient determination method for nonvolatile chemicals in biological tissues. Fundam Appl Toxicol 2017. [Google Scholar]
- A R Amaro, G G Oakley, U Bauer, H P Spielman, L W Robertson. Metabolic activation of PCBs to quinones: reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol 2016. [Google Scholar]
- . American Chemistry Council. HPV data summary and test plan for Hex- abromocyclododecane (HBCD). Arlington, VA: American Chemistry Council; Report AR201-13459A submitted to the US-EPA by the Brominated Flame Retardant Industry Panel. 2016. [Google Scholar]
- D T Szabo, J J Diliberto, H Hakk, J K Huwe, L S Birnbaum. Toxicokinetics of the flame retardant hexabromocyclododecane gamma: effect of dose, timing, route, repeated exposure, and metabolism. Toxicol Sci 2015. [Google Scholar]
- L T Van Der Ven, A Verhoef, T Van De Kuil, W Slob, P E Leonards, T J Visser. A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in Wistar rats. Toxicol Sci 2016. [Google Scholar]
- B H Wilford, M Shoeib, T Harner, J Zhu, K C Jones. Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: implications for sources and exposure. Environ Sci Technol 2015. [Google Scholar]
- F Wu, T K Takaro. Childhood asthma and environmental interventions. Environ Health Perspect 2017. [Google Scholar]
- Q Xian, K Ramu, T Isobe, A Sudaryanto, X Liu, Z Gao. Levels and body distribution of polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecanes (HBCDs) in freshwater fishes from the Yangtze River China. Chemosphere 2017. [Google Scholar]
- Takashi Yamada, T Yuhki, Y Yasuhiro. Isolation ofPseudomonassp. Strain HB01 Which Degrades the Persistent Brominated Flame Retardant γ-Hexabromocyclododecane. Biosci, Biotechnol Biochem 2009. [Google Scholar] [Crossref]
- C C Yu, Y H Atallah, R Van Bommel, B N Zegers, A Mets, T Hamers, . Levels of hexabromocyclododecane in harbor porpoises and common dolphins from Western European seas, with evidence for stereoisomer specific biotransformation by Cyt-P450. Environ Sci Technol 2013. [Google Scholar]
- D Jérémie, KD Lebrun, L Jesus, M Rouillac, A Ravelli, J Guenne. Single and combined effects of insecticides on multi-level biomarkers in the non-target amphipod Gammarus fossarum exposed to environmentally realistic levels. Aquatic Toxicol 2014. [Google Scholar]
- K Dalhoff, AMB Hansen, JJ Rasmussen, A Focks, W Bjarne, N Strobel. Linking Morphology, Toxicokinetic, and Toxicodynamic Traits of Aquatic Invertebrates to Pyrethroid Sensitivity. Environ Sci Technol 2016. [Google Scholar]
- NA Munz, Q Fu, C Stamm, J Hollender. Internal Concentrations in Gammarids Reveal Increased Risk of Organic Micropollutants in Wastewater-Impacted Streams. Environ Sci Technol 2018. [Google Scholar] [Crossref]
- D Englert, JP Zubrod, M Link, S Mertins, R Schulz, M Bundschuh. Does Waterborne Exposure Explain Effects Caused by Neonicotinoid-Contaminated Plant Material in Aquatic Systems?. Environl Sci Technol 2017. [Google Scholar] [Crossref]
- MM Willming, CR Lilavois, MG Barron, S Raimondo. Acute Toxicity Prediction to Threatened and Endangered Species Using Interspecies Correlation Estimation (ICE) Models. Environ Sci Technol 2016. [Google Scholar] [Crossref]
- A Franco, OR Price, S Marshall, O Jolliet, PJV den Brink, A Rico. Toward refined environmental scenarios for ecological risk assessment of down-the-drain chemicals in freshwater environments. Integr Environ Assess Manag 2017. [Google Scholar] [Crossref]
- CU Johnston, LN Clothier, DM Quesnel, LM Gieg, G Chua, PM Hermann. Wildering. Embryonic exposure to model naphthenic acids delays growth and hatching in the pond snail Lymnaea stagnalis. Chemosphere 2016. [Google Scholar]
- R.S. Prosser, S.R. de Solla, E.A.M. Holman, R. Osborne, S.A. Robinson, A.J. Bartlett. Sensitivity of the early-life stages of freshwater mollusks to neonicotinoid and butenolide insecticides. Environ Pollut 2016. [Google Scholar] [Crossref]
- TH Miller, GL. McEneff, LC Stott, SF Owen, NR Bury, LP Barron. Assessing the reliability of uptake and elimination kinetics modelling approaches for estimating bioconcentration factors in the freshwater invertebrate, Gammarus pulex. Sci Total Environ 2016. [Google Scholar] [Crossref]
- F Alonzo, T Hertel-Aas, A Real, E Lance, L Garcia-Sanchez, C Bradshaw. Population modelling to compare chronic external radiotoxicity between individual and population endpoints in four taxonomic groups. J Environ Radioactivity 2016. [Google Scholar] [Crossref]
- M Brinkmann, TG Preuss, H Hollert. Advancing In Vitro-In Vivo Extrapolations of Mechanism-Specific Toxicity Data Through . Toxicokinetic Modeling 2018. [Google Scholar]
- Introduction
- Toxicokinetics
- Clinical veterinary toxicology
- Models and methods for in vitro toxicity
- Preclinical drug development
- Toxicity and safety evaluation of pesticides
- Toxicity and Safety Testing
- Modeling Toxicity
- Toxicokinetics Modeling
- Orally delivered nanoparticle drug delivery systems for dental application and their toxicity on system organs
- Principles of Toxicokinetics
- Toxicology Testing
- Applications of Toxicokinetics
- Conclusion
- Conflicts of Interest
- Source of Funding
How to Cite This Article
Vancouver
Sandhya . Toxicokinetics an essential tool in drug discovery: A review article [Internet]. IP Int J Compr Adv Pharmacol. 2025 [cited 2025 Sep 06];6(1):5-9. Available from: https://doi.org/10.18231/j.ijcaap.2021.002
APA
Sandhya, (2025). Toxicokinetics an essential tool in drug discovery: A review article. IP Int J Compr Adv Pharmacol, 6(1), 5-9. https://doi.org/10.18231/j.ijcaap.2021.002
MLA
Sandhya, . "Toxicokinetics an essential tool in drug discovery: A review article." IP Int J Compr Adv Pharmacol, vol. 6, no. 1, 2025, pp. 5-9. https://doi.org/10.18231/j.ijcaap.2021.002
Chicago
Sandhya, . "Toxicokinetics an essential tool in drug discovery: A review article." IP Int J Compr Adv Pharmacol 6, no. 1 (2025): 5-9. https://doi.org/10.18231/j.ijcaap.2021.002
