- Received August 10, 2024
- Accepted September 21, 2024
- Publication November 26, 2024
- Visibility 2 Views
- Downloads 0 Downloads
- DOI 10.18231/j.ijcaap.2024.035
-
CrossMark
- Citation
Advances in neurotransmitter detection and modulation: Implications for neurological disorders
Introduction
The central nervous system (CNS) is the primary control centre for all activities in the human body, processing information received from and transmitted to the peripheral nervous system (PNS). This intricate system governs various physiological functions, including muscle control, secretion regulation, and organ function, through signal conduction between neurons. These signals are passed across specialized junctions known as synapses, where communication is mediated by chemical messengers called neurotransmitters (NTs). This process, known as synaptic transmission or neurotransmission, forms the basis for the CNS’s ability to control smooth, skeletal, and cardiac muscles, manage bodily secretions, and regulate organ function.[1]
NTs are endogenous chemical messengers that serve as the primary mediators of communication within the nervous system. These tiny molecules play an important role in transferring and enhancing impulses between neurones and other cell types, such as muscle or gland cells. They regulate and communicate sensory, motor, and integrative neural information, impacting a wide range of body activities, such as emotions, thoughts, memories, movements, and sleep patterns. In addition to these roles, NTs also play an essential part in the regulation of neuronal growth, differentiation, and survival, making them critical for the overall functioning of the brain.[2]
The significance of NTs in maintaining the proper functioning of the nervous system cannot be overstated. Their roles extend beyond simple signal transmission; they are integral to maintaining homeostasis in the brain, allowing neurons to communicate effectively and ensuring the body responds appropriately to external and internal stimuli. The diversity of NTs reflects the complexity of the CNS, where each neurotransmitter has specific functions and targets. The balance and homeostasis of these chemicals are vital to normal brain function. When this balance is disrupted, it can lead to a range of physical, psychotic, and neurodegenerative diseases.[3], [4] For instance, an excess or deficiency of specific NTs can result in mood disorders like depression or anxiety, while neurodegenerative conditions such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) have also been linked to neurotransmitter imbalances. Over the past century, extensive research has led to the identification of hundreds of NTs, classified into two broad categories: canonical and noncanonical NTs. Canonical NTs include amino acids, monoamines acetylcholine, purines, soluble gases and neuropeptides. These NTs have long been recognized for their role in transmitting signals within the CNS and PNS and have been studied extensively for their involvement in various neurological functions and disorders. Glutamate, for example, is the primary excitatory neurotransmitter in the brain, while GABA is the major inhibitory neurotransmitter. Dopamine is well-known for its role in reward, motivation, and motor control, while serotonin is involved in mood regulation, sleep, and appetite.[5], [6], [4]
In contrast, noncanonical NTs have only recently gained attention and are less understood compared to their canonical counterparts. These include molecules such as exosomes, steroids, and D-aspartic acid, which, although not traditionally considered NTs, have been found to play critical roles in neuronal communication and regulation.[7] Exosomes, for instance, are small vesicles that carry various molecular signals between cells, influencing processes such as neuroplasticity and neuroinflammation. Steroids, which are typically recognized for their role in hormonal regulation, have been shown to modulate neurotransmission, particularly in stress responses. D-aspartic acid, a non-protein amino acid, is involved in synaptic plasticity and has been linked to neurodevelopmental processes. Given the essential functions that NTs perform in the brain and nervous system, it is no surprise that abnormalities in their levels and functions can have severe consequences. Dysregulation of neurotransmitter systems is implicated in a wide array of neurological and psychiatric conditions.[8] For example, a deficiency in dopamine is a hallmark of PD, a neurodegenerative disorder characterized by motor impairment, while an imbalance in serotonin levels is associated with mood disorders such as depression. Similarly, abnormal glutamate signaling is implicated in neurodegenerative diseases like AD, where excitotoxicity (overactivation of glutamate receptors) leads to neuronal damage and cognitive decline.
Understanding the complex roles of NTs and their involvement in these diseases is crucial for developing effective therapeutic strategies. In recent years, there has been significant progress in the development of novel methods for detecting NTs, which has opened new avenues for research. These detection methods include advanced imaging techniques, biosensors, and electrochemical approaches that allow for the real-time monitoring of neurotransmitter levels in different regions of the brain. Such advancements have enhanced our understanding of neurotransmitter dynamics in both healthy and diseased states. Furthermore, efforts to modulate neurotransmitter levels as a treatment strategy have gained significant attention.[9], [10] Pharmacological agents that target specific neurotransmitter systems, such as SSRIs for depression or dopamine agonists for PD, have become standard treatments for various neurological and psychiatric conditions. Additionally, non-pharmacological approaches, such as deep brain stimulation and transcranial magnetic stimulation, are being explored as ways to restore neurotransmitter balance and improve symptoms in patients with neurological disorders.[11]
In this context, this review aims to provide a comprehensive overview of the known canonical and noncanonical NTs, emphasizing their roles in neurological and neurodegenerative diseases. Additionally, novel detection methods for NTs will be discussed, alongside potential strategies for modulating neurotransmitter levels to restore homeostasis and treat associated neurological conditions. Through a deeper understanding of neurotransmitter systems, we can better address some of the most pressing challenges in neuroscience and neuropharmacology.
Neurotransmitters
NTs are molecules that play a critical role in amplifying, transmitting, and converting signals within cells, making them essential for brain function, behavior, and cognition. Since their discovery in 1921, more than 200 of these chemical messengers have been identified, although the precise number remains unknown. This uncertainty is largely due to the continuous discovery of new biomolecules exhibiting neuroactive properties, which are added to the growing list of recognized NTs. [12]
To be categorised as a neurotransmitter, a chemical must satisfy several fundamental criteria: (i) it must be created and released by the same neurone, with storage taking place at the presynaptic terminal; (ii) it should cause a particular response in the postsynaptic neurone; (iii) its exogenous administration should produce the same effect as its endogenous counterpart; and (iv) there must be a specific mechanism to terminate its action on the postsynaptic cell.[13]
Over time, various types of NTs have been identified and studied for their influence on brain health. NTs are broadly classified into two categories: canonical and noncanonical. Canonical NTs include small molecules that are widely recognized as NTs, while noncanonical NTs represent neuroactive compounds that have only recently been identified and are still subject to ongoing research and debate[14]
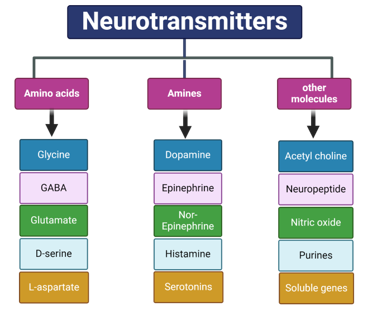
Canonical Neurotransmitters
Several types of NTs have been identified, each with distinct functions and areas of production within the brain. From a chemical standpoint, NTs are commonly grouped into categories such as amino acids, amines, and other molecules. Functionally, they can also be classified as either excitatory (positive) or inhibitory (negative) NTs, or according to whether they act centrally in the brain or peripherally in other parts of the nervous system. The next sections will explore the main canonical NTs, categorized based on their chemical nature and functions.[6]
Amino acid
Amino acid NTs are vital to the CNS, playing crucial roles in brain function and the development of various disorders. Among these are α-amino acids, such as glutamate and glycine, and GABA, which are engaged in important brain functions and contribute to the development of certain neurological disorders.[15]
Glutamate is the principal excitatory neurotransmitter in the CNS, synthesised from glutamine and acting as a precursor to GABA. Presynaptic neurones release it into the synaptic cleft, activating NMDA and AMPA receptors. This activation promotes the inflow of calcium and sodium ions into postsynaptic neurones. However, high glutamate can cause overactive calcium signalling in postsynaptic neurones, resulting in hyperactive neuronal firing and excitotoxicity. This excitotoxicity has been linked to neurological illnesses such as ALS and PD.[15], [16]
Astrocytes regulate extracellular glutamate levels through both release and absorption processes. They take excess glutamate from the synaptic cleft and convert it to glutamine, which is subsequently delivered back to the presynaptic neurones. Furthermore, a part of the glutamate is released into the extracellular space via multiple mechanisms. This cycle helps control glutamate homeostasis in the tripartite glutamatergic synapse, hence preventing detrimental excitotoxicity.[17], [18]
GABA, another key amino acid neurotransmitter, is produced by the enzyme glutamate decarboxylase or synthesized by commensal gut microbiota. Interestingly, although GABA is known as the primary inhibitory neurotransmitter in the brain, early studies have shown that it initially exhibits excitatory properties, Inducing stimulation rather than inhibition in certain brain areas. A lack of GABA is linked to neuronal hyperexcitability, contributing to conditions such as anxiety and epilepsy. [19], [20], [21]
Glycine is the primary inhibitory neurotransmitter in the spinal cord, whereas in the brainstem and medulla, it acts as a neurotransmitter and co-agonist at NMDA receptors with glutamate. Glycine, like GABA, shows excitatory activity throughout early development before switching to an inhibitory function in maturity. It is responsible for a variety of tasks, including voluntary muscular control, sensory processing, and regulation of the auditory, cardiovascular functions.[22], [23]
D-serine is another neurotransmitter, released by glial cells, whose role in higher organisms has been only recently explored. It is synthesized from L-serine by the enzyme serine racemase, particularly in regions of the brain rich in NMDA-glutamate receptors. Another neurotransmitter, L-aspartate, has been a subject of debate due to conflicting findings regarding its localization and function. Some studies suggest that L-aspartate acts as a neurotransmitter in the visual cortex and cerebellum, while others propose that it functions both as a neurotransmitter and a neuropeptide-like modulator in the hippocampus.[24]
Amines
Monoamines are a group of NTs that play critical roles in regulating motor functions, emotional responses, motivation, and behavior. These NTs are synthesized by presynaptic neurons and act by binding to specific receptors on the postsynaptic membrane(24). Any excess monoamines remaining in the synaptic cleft are degraded by enzymes such as MAO or COMT. Dysregulation of this process is linked to several severe neurological disorders, including AD, PD, HD, and schizophrenia.[25]
Dopamine is one of the most important neurotransmitters in mammals, impacting almost all physiological activities in the CNS, either directly or indirectly. It is created and released by dopaminergic neurones, which are mostly found in the substantia nigra pars compacta and ventral tegmental region. Dopamine is necessary for homeostasis and acts as a precursor to other catecholamines, including norepinephrine and epinephrine. Any imbalance in dopamine levels can lead to many behavioural and neurological diseases, including drug addiction, schizophrenia, Parkinson's disease, and hypertension.[26]
Serotonin is another vital monoamine neurotransmitter, known for its role in modulating a wide range of physiological processes. It influences sleep-wake cycles, gastrointestinal secretion, respiration, vasoconstriction, behavior, and overall neurological function.[27] Enterochromaffin cells in the gut create the vast majority of serotonin, with the tryptophan hydroxylase enzyme playing a critical role in its production. Serotonin has a wide-ranging effect on the CNS, regulating other NTs by inhibiting dopamine release, modulating glutamate and GABA transmission, and influencing glutamate release in various brain regions, such as inhibiting glutamate release in the frontal cortex while increasing it in the prefrontal cortex. [28]
Epinephrine and norepinephrine are monoamines that operate as both neurotransmitters and hormones. Norepinephrine neurones are mostly found in the locus coeruleus and send signals to many regions of the brain, including the limbic system, which is involved in emotional regulation.[29] While epinephrine is also produced by specific neurons in the brain, its role as a neurotransmitter remains less understood. Both of these NTs are crucial in the body's response to stress and are involved in the "fight or flight" response.[30]
Histamine is another monoamine signaling molecule that functions as a neurotransmitter within the CNS. It is involved in various physiological processes, including regulating sleep-wake cycles, immune responses, and inflammatory reactions. Although its role in the CNS is not as extensively studied as other monoamines, histamine is increasingly recognized for its importance in maintaining overall neurological health and function. In summary, monoamines are fundamental NTs in the CNS, playing crucial roles in behavior, cognition, and physiological homeostasis. Dysregulation of monoamine systems is implicated in numerous neurological and psychiatric conditions, highlighting the need for continued research into their functions and therapeutic potential.[31]
Other molecules as a neurotransmitters
In addition to the above-mentioned NTs, numerous additional compounds have been discovered as NTs. Acetylcholine, the first chemical to be characterised and recognised as a neurotransmitter in the PNS, is one of the best-studied. Acetylcholine is produced by post-ganglionic neurones in the parasympathetic nervous system and is required for muscle contraction at the neuromuscular junction. Acetylcholine is important in the CNS for awareness and cognitive activities such as attention, learning, memory, voluntary movement, and sleep. Cholinergic neurones, which produce acetylcholine, are dispersed across various brain areas, including the striatum, cranial nerves, and vestibular nuclei.[32]
Acetylcholine is stored in synaptic vesicles within cholinergic presynaptic neurones and, after neuronal depolarisation, is released into the synaptic cleft to permit neurotransmission via acetylcholine receptors. It serves as a neuromodulator in several areas of the forebrain, influencing motor and cognitive activities via cortico-striato-thalamocortical circuits. Acetylcholine imbalances have been associated to neurological illnesses such as Alzheimer's disease, Parkinson's disease, hypertension, schizophrenia, myasthenia gravis, as well as learning, attention, memory, and sleeping difficulties.[33], [34]
In addition to acetylcholine, several compounds are recognised as NTs. These include purines like adenosine triphosphate (ATP), soluble gases known as gasotransmitters (e.g., carbon monoxide [CO], nitric oxide [NO], and hydrogen sulphide [H2S]), and different neuropeptides such as somatostatin, substance P, and neuropeptide Y. A brief description of a specific example from each category suggests below.
ATP, sometimes known as the "energy currency" of the cell, is required for several key activities within organisms and cells, such as intracellular signalling, active transport, muscular contraction, and DNA/RNA synthesis. Synaptic transmission, an energy-intensive mechanism, requires ATP at the presynaptic terminal to sustain ion gradients that assist shuttle NTs into vesicles and prepare them for release by exocytosis. at the central nervous system, ATP is recognised as an excitatory neurotransmitter at neuronal synapses. Insufficient ATP release has been associated to different dysfunctions, including brain traumas, strokes, PD and AD.[35], [36]
Among soluble gases, nitric oxide (NO) stands out as a well-established neurotransmitter and signaling molecule, crucial in regulating synaptic plasticity. NO also plays a role in modulating the biosynthesis of D-serine, another important neurotransmitter. Serine racemase, the enzyme responsible for converting L-serine to D-serine, is physiologically nitrosylated by NO, which inhibits its activity and lowers the conversion rate. NO is produced in response to NMDA receptor activation and diffuses to D-serine-producing cells, providing a form of feedback inhibition. Given its wide-reaching effects, NO is crucial for maintaining proper synaptic function and neurochemical balance.[37], [38]
Neuropeptide Y (NPY) is one of the most extensively expressed NTs in the nervous system, as well as the most abundant peptide in the mammalian brain. NPY is implicated in a wide range of biological activities, including cortical excitability, stress response, food intake control, circadian rhythms, and cardiovascular function. Abnormal regulation of NPY is linked to a range of neurological conditions, such as epilepsy, and is implicated in other disorders like obesity, anxiety, and mood disturbances. NPY's widespread influence makes it an important target for research into neurophysiological and neuropsychiatric disorders.[39], [40]
Noncanonical Neurotransmitters
Noncanonical NTs have recently garnered increasing attention in scientific research and debate due to their complex roles in brain function, intercellular communication, and potential involvement in neurodegenerative diseases.[41]. Among the most notable of these nontraditional NTs are exosomes, which are small bilayered extracellular vehicles (EVs) that serve as long-range messengers. These vesicles have been implicated in a range of critical functions, including the control of growth and development, intercellular communication, antigen presentation, inflammation, and cancer. Although exosomes were first researched for these biological tasks, new research indicates that they also have a role in neurotransmission and synaptic control.[42]
Exosomes share functional similarities with synaptic vesicles but differ in that they are released into the extracellular space by their parent cells rather than within synapses. Exosomes contain diverse molecular cargo, including proteins, lipids, and RNAs that influence synaptic plasticity and neuronal communication. In addition to their role in synaptic plasticity, neuronal exosomes have been proposed as direct mediators of neurotransmission. [43] Exosomes, like classical neurotransmitters, are released by presynaptic neurones in response to action potentials. They can carry neuropeptides and ligands that activate G protein-coupled receptors (GPCRs), initiating intracellular signalling cascades.[44] Exosomes, once released, can raise intracellular calcium (Ca2+) levels in postsynaptic neurones by promoting Ca2+ release from the endoplasmic reticulum via inositol 1,4,5-triphosphate (IP3) receptors and activating calcium channels on the cell membrane. This rise in Ca2+ causes quick reactions in postsynaptic neurones and allows for long-term changes in synaptic strength via controlling the number of receptors and the activity of certain ion channels.[45]
Exosomes also contribute to a variety of physiological processes in the CNS, such as neurone regeneration, synapse maintenance, and immunological responses. Their participation in neurodegenerative illnesses including AD and PD has prompted worries regarding their possible harmful impact.[46] Certain exosomal proteins, including Alix and Flotillin-1, have been linked to the propagation of AD and PD. These proteins may facilitate the spread of misfolded proteins like amyloid-beta in AD or alpha-synuclein in PD, contributing to the progression of these diseases.[47]
Another noncanonical neurotransmitter class is steroids, which exhibit neurotransmitter-like properties. Steroids can signal within the brain and the nucleus, activating intracellular signaling cascades and modulating calcium release. [48] The neuroactive effects of steroids are not limited to their role in the endocrine system; they also play a part in synaptic transmission, influencing neuronal excitability and synaptic plasticity.[49]
D-aspartic acid, a noncanonical neurotransmitter, is another molecule with potential neuroactive effects. This amino acid has been found in nervous tissues of several animal species as well as in human brains.[50] Although its exact role as a neurotransmitter is still being studied, D-aspartic acid has been suggested to participate in neurotransmission and may contribute to neural development and plasticity.[51]
The exploration of noncanonical NTs, such as exosomes, steroids, and D-aspartic acid, continues to expand our understanding of brain function and neural communication. These molecules challenge traditional definitions of neurotransmission and open new avenues for research, particularly concerning their roles in neurodegenerative diseases. Understanding the mechanisms through which these molecules influence neuronal signaling may provide valuable insights into potential therapeutic targets for neurological and psychiatric disorders.[52]
Neurotransmitter Disorders of the CNS
NTs are integral to various diseases, such as epilepsy and multiple sclerosis (MS), which arise from disruptions in NT metabolism. These disruptions can affect NTs like amino acids, monoamines, cholinergic transmission, purines, and others. These imbalances may be genetic, or they can develop over time due to issues like impaired neuronal receptors, faulty intracellular signaling, vesicle release problems, or other synaptic dysfunctions.[53]
Epilepsy
Epilepsy is a devastating neurological illness marked by seizures produced by a sudden and transient synchronisation of neuronal activity. The illness is largely impacted by an imbalance of excitatory and inhibitory neurotransmitters, especially glutamate and GABA. Glutamate is the primary excitatory neurotransmitter in the CNS, and excess activity promotes neuronal excitability, which contributes to seizure onset. To address this, contemporary anti-seizure drugs target ion channels, transporters, and receptors to restore balance between these NTs, resulting in symptomatic relief.[54], [55]
Multiple sclerosis
Multiple sclerosis (MS) is a chronic, autoimmune-driven inflammatory disease that affects the central nervous system. Although the specific aetiology is unknown, it involves both genetic and environmental factors. MS is characterised by demyelination, astroglial proliferation, and neurodegeneration in the CNS. One key feature of MS is glutamate excitotoxicity, which is caused by high extracellular glutamate levels. Research has found that single nucleotide polymorphisms (SNPs) in glutamate transporter genes can disrupt the expression of EAAT1/2 transporters, which regulate glutamate uptake. This dysregulation leads to excitotoxicity and worsens tissue damage in MS.[56], [57]
GABA, another key NT, has also been implicated in MS. Lower GABA+ levels are common in people with relapsing-remitting MS (RRMS), and aberrant GABAergic neurotransmission may contribute to cognitive impairment. GABAergic interneurons help stabilize the brain's inhibitory neural network by providing recurrent inhibition to pyramidal neurons. When this network is disrupted, cognitive function suffers, particularly in individuals with RRMS.[58]
Genetic studies have identified several genes linked to MS, including those coding for human leucocyte antigens (HLA) class I and II, T-cell receptor β, and other immune-related molecules like CTLA4, ICAM1, and SH2D2A. Understanding how neurotransmitter imbalances relate to MS pathology is critical in developing more targeted and effective treatments for the disease.[59]
Autism
Autism spectrum disorders (ASD) are complex neurodevelopmental and neurobehavioral conditions marked by challenges in social interaction, communication, restrictive behaviors, and altered sensory processing. The heterogeneity of ASD suggests multiple potential underlying causes, with neurochemical imbalances playing a key role in its pathophysiology. Variable levels of GABA and glutamate in children with ASD contribute to an imbalance of excitatory and inhibitory neurotransmission.[60] Prenatal exposure to GABAA receptor inhibitors has been linked to ASD-like behaviors in offspring. Additionally, glutamatergic circuits connecting the frontal regions and the striatum are involved in regulating compulsive behaviors, such as stereotypy, often seen in ASD.[61] changes in genes like GRIN2A and GRIN2B have been connected with these illnesses, whereas glutamatergic dysregulation is also linked to changes in genes involved in synapse development and maintenance.
Monoamine neurotransmitter abnormalities are also implicated in ASD, with disruptions in dopamine, norepinephrine, and serotonin balance contributing to altered sleep, mood, and behavior. Autistic people have diminished dopamine release in the prefrontal cortex and impaired neuronal responses in the nucleus accumbens. A recent hypothesis proposes that ASD behaviors may result from dysfunctions in the midbrain dopaminergic system, where mesocorticolimbic (MCL) circuit dysfunction leads to social deficits, and nigrostriatal (NS) circuit dysfunction contributes to stereotyped, repetitive behaviors.[62]
Alzheimer's disease
AD is a neurodegenerative ailment that predominantly affects the neocortex, characterised by gradual memory loss, behavioural abnormalities, and a high death rate. The specific aetiology of AD remains unknown; however, its pathology is intimately related with the formation of Aβ plaques and hyperphosphorylated tau protein clumps. Other contributing factors include synaptic protein changes, neurotransmitter loss, oxidative stress, mitochondrial dysfunction, calcium deregulation, inflammation, and cerebral disease. Several hypotheses attempt to explain AD pathogenesis. The amyloid cascade hypothesis suggests that increased production of amyloidogenic Aβ42 leads to neuronal death and synaptic loss. The calcium hypothesis of AD proposes that neurodegeneration is driven by disruptions in cellular calcium homeostasis, contributing to synaptic dysfunction and cell death.
Neurotransmitter imbalances are key to understanding AD's cognitive and behavioral symptoms. Cholinergic dysfunction, marked by acetylcholine deficits, is linked to cognitive decline and exacerbates amyloid-beta and tau pathology. Damage to cholinergic signaling results in impaired memory and cognition. Dopaminergic deficits are also tied to cognitive impairments and may contribute to AD-related behavioral changes, such as apathy and mood disturbances, as dopamine plays a role in the brain's reward and motivation circuits[63], [64]
Serotonergic dysfunction in AD has been implicated in neuropsychiatric symptoms, including depression and agitation. Restoring serotonin levels has been shown to alleviate both cognitive and behavioral symptoms, making it a potential target for AD therapy.[63] Addressing these NT deficits might offer therapeutic strategies for managing AD's complex neurodegenerative profile.
Parkinsons disease
Parkinson's disease is a progressive neurodegenerative disorder characterised by the degradation of dopaminergic neurones in the substantia nigra pars compacta, which results in dopamine insufficiency in the striatum and the production of Lewy bodies. This results in a range of symptoms, including motor disturbances, hyposmia (reduced sense of smell), autonomic dysfunction, sleep disorders, and psychiatric or cognitive impairments. In addition to dopamine, other NTs like glutamate, GABA, serotonin, histamine, acetylcholine, and epinephrine are also affected in PD. [64], [65]
One emerging aspect of PD pathology is the disruption of glutamate homeostasis in the striatum. Inflammatory processes contribute to astrocytic glutamate excitotoxicity by altering the expression of glutamate transporters and receptors. [66] The accumulation of α-synuclein, a protein associated with PD, increases the presynaptic release of glutamate in a calcium-dependent manner, which activates extra synaptic NMDA receptors and leads to neuronal damage. Elevated glutamate levels also stimulate AMPA receptors, further promoting glutamate release. Additionally, α-synuclein enhances the mobilization of glutamate-containing vesicles, causing overstimulation of mGluR5 receptors, contributing to excitotoxicity and neuronal injury. [67]
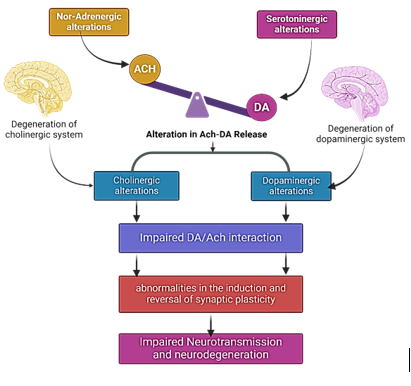
cholinergic hyperactivity due to acetylcholine (Ach) excess, dopaminergic deficits from dopamine (DA) loss, disrupted DA/Ach interactions causing motor issues, and impaired synaptic plasticity, affecting learning and motor adaptation.
Huntington's disease
Huntington's is a neurodegenerative disorder caused by an expansion of CAG trinucleotide repeats in the first exon of the huntingtin gene, leading to polyglutamine expansions and toxic aggregation of the huntingtin protein in neurons, particularly in striatal and cortical motor regions, as well as prefrontal areas. The abnormal body movements observed in HD arise from disruptions in the balance between the direct and indirect pathways of motor control, accompanied by altered levels of several NTs in the striatum.
The most severe neurodegeneration occurs in the caudate and putamen, areas known for their rich dopaminergic innervation and high concentrations of dopamine receptors. In the early stages of HD, the heightened thalamocortical glutamatergic signaling contributes to hyperkinetic (excessive) movements. As the disease progresses, hypokinesia (reduced movement) develops as both the direct and indirect pathways become compromised. Research suggests that the interaction between dopamine and glutamate pathways, particularly through the activation of D1 receptors, may exacerbate neurotoxicity, further contributing to the progression of the disease.[68], [69], [70].
Schizophrenia
Schizophrenia is a severe neurodevelopmental condition with unknown origins that is frequently diagnosed during adolescence. It manifests as a variety of symptoms, including hallucinations, delusions, social isolation, lack of desire, and cognitive deficits. Cognitive deficiencies, such as difficulties with working memory, executive function, learning, long-term memory, and sensory perception, are substantial predictors of long-term functional outcome in schizophrenia.[71]
The cognitive symptoms of schizophrenia are thought to stem from primary deficits in NMDA receptor glutamatergic signaling, particularly in layer 3 pyramidal neurons of the prefrontal cortex. These deficiencies impede executive cognitive skills as well as working memory maintenance. Furthermore, abnormalities in GABA neurotransmission may lead to working memory problems. Working memory is strongly related with gamma frequency oscillations in the prefrontal cortex, which are decreased in persons with schizophrenia. Reduced GABAergic neurotransmission is linked to increased dopamine synthesis, and abnormalities in both presynaptic and postsynaptic dopaminergic systems have been associated with the onset of schizophrenia. Dysregulation of the dopaminergic system is considered a major factor in the etiology of the disorder. [72], [73], [74], [75], [76]
Depression
Depression is a complex and multifaceted disorder associated with various biological mechanisms beyond behavioral factors. This involves inflammatory reactions, hypothalamic-pituitary-adrenal axis dysregulation, abnormalities in sympathetic and parasympathetic and endothelial dysfunction accompanied by platelet activation. Neurobiological research has indicated that depression is associated with neuronal shrinkage in cortical and limbic brain areas, as well as impaired brain connections and network function. These changes are attributed to structural, functional, and neurochemical deficits, particularly involving dysfunctions in the GABA and glutamate systems.[19], [77]
One hypothesis regarding depression emphasizes the role of monoamine NTs. It suggests that low levels of serotonin, dopamine, and norepinephrine are associated with depression, and these neurotransmitter levels can be increased by antidepressant medications. However, research on monoamine levels has produced mixed results, with some studies contradicting this link. On the other hand, alterations in GABAergic and catecholaminergic pathways have shown more consistent diagnostic value in understanding depression. [78]
Amyotrophic lateral sclerosis
ALS is a chronic neurological disease with a complicated pathophysiology. Initially thought to be a pure motor neurone disease, it has since been recognised as a multisystem condition with clinical, inherited, and neuropathological fluctuation. ALS causes motor neurone degeneration, muscular atrophy, paralysis, and severe metabolic dysregulation. ALS's mechanisms comprise ROS-associated oxidative stress, dysfunction of the mitochondria, compromised homeostasis, axonal and vesicular transport dysfunction, excitotoxicity of glutamate, proteostatic deficits, altered RNA metabolism, low Ca2+ buffering capacity, a high number of AMPA receptors in motor neurones, neuroinflammation, and neurotrophin depletion.[79], [80]
Approximately 2% of ALS cases have a genetic component, including mutations in SOD1 and other prevalent risk factors. Treatments focused on reducing NT concentrations have been developed, with riluzole being the sole disease-modifying medicine licensed in the majority of European nations. This medication has been shown to increase patient lifespan by 3 to 6 months, however it includes adverse effects such as nausea, diarrhoea, exhaustion, disorientation, and liver damage.[81], [82]
Neurotransmitters Detection
Diagnosing brain disorders chemically presents considerable challenges due to the difficulty of analyzing brain chemistry in living organisms and the protective blood-brain barrier that limits direct access to the CNS. Despite these obstacles, early detection of NTs is crucial for the prevention and management of neurological disorders. Recent research has aimed to overcome these challenges by developing and refining tools for the direct detection of chemical biomarkers associated with these conditions. [9], [83]
Several advanced techniques have been employed to facilitate the diagnosis and detection of NTs. Electrochemical methods are used to measure changes in electrical properties that occur when NTs interact with specific sensors. Fluorescence Resonance Energy Transfer (FRET) utilizes the transfer of energy between two fluorescent molecules to detect specific biomarkers, offering high sensitivity and specificity. Chemiluminescence involves the emission of light resulting from chemical reactions, making it useful for detecting low concentrations of NTs. Chromatography separates NTs based on their chemical properties, which aids in their identification. Mass Spectrometry provides precise detection by analyzing the mass-to-charge ratio of NTs. Capillary Electrophoresis separates NTs based on their size and charge using an electric field, allowing for accurate analysis. Surface-Enhanced Raman Spectroscopy (SERS) enhances Raman scattering to detect NTs even at very low concentrations. Near-Infrared (NIR) Biosensing employs NIR light to analyze NTs, while Microdialysis allows for the collection and measurement of NTs from the extracellular fluid in the brain.[84]
In addition to these techniques, advancements in nanomaterial-based detection systems are showing great promise. Carbon-Based Nanosensors use carbon materials to achieve high sensitivity and selectivity in detecting NTs. Metal-Based Nanosensors employ metals to enhance detection capabilities, while Metal-Oxide-Based Nanosensors offer high performance in identifying various NTs. Polymer-Based Nanosensors provide flexibility and customization for specific applications, and Enzyme-Based Nanosensors use enzymes to selectively interact with NTs, allowing for precise measurements. These innovations are significantly improving the ability to detect and monitor NTs, thereby enhancing the diagnosis and management of neurological disorders. [85], [86]
Modulation of Neurotransmitters and Neurotransmitter Transporters as a Therapeutic Strategy
Neurotransmitter transporters (NTTs) are integral to maintaining the delicate balance of within the brain. These transporters are responsible for regulating extracellular NT concentrations by facilitating their uptake into cells, which helps in limiting receptor activation and ensuring appropriate downstream signaling. Consequently, NTTs have emerged as promising targets for therapeutic interventions aimed at treating a range of neurological and neuropsychiatric disorders.[87]
Some of the important NTTs are glutamate transporters, notably the glutamate-aspartate transporter and glutamate transporter-1, as well as their human homologs, excitatory amino acid transporters 1 and 2. These transporters are mostly expressed in astrocytes and serve an important function in controlling extracellular glutamate levels.[88] By facilitating the uptake of glutamate from the synaptic cleft, these transporters prevent excessive accumulation and thereby mitigate the risk of excitotoxicity—a condition where excessive glutamate causes neuronal damage and death.[89] Enhancing the production and function of these transporters has been investigated as a therapeutic approach. Several pharmacological drugs, including β-lactam antibiotics, selective oestrogen receptor modulators, growth factors, histone deacetylase inhibitors, and translational activators, have demonstrated the ability to modulate these transporters. For instance, β-lactam antibiotics like ceftriaxone have been reported to upregulate GLT-1 expression, thereby providing neuroprotection in conditions such as ALS and other neurodegenerative diseases.[90]
Monoamine transporters (MATs) are another crucial target for therapeutic interventions. These transporters regulate the levels of key monoamines, including serotonin, dopamine, and norepinephrine, which are implicated in a variety of neuropsychiatric disorders. MATs are targeted by various drugs, including antidepressants and substances of abuse. Such as SSRI act by inhibiting serotonin reuptake, therefore increasing its availability in the synaptic cleft and reducing mood and anxiety symptoms [91] Caffeine, a well-known psychoactive substance, also modulates NT systems, particularly in the mesocorticolimbic brain regions, which are involved in reward and addiction pathways. While moderate caffeine consumption can enhance dopaminergic signaling and offer neuroprotective benefits, excessive intake may lead to neurotoxicity, negative behavioral effects, and other health issues. Therefore, careful dosing and monitoring are essential when using caffeine as a therapeutic agent.[92] Recent research has highlighted the influence of gut microbiota on NT regulation through the gut-brain axis. Prebiotics and probiotics have emerged as potential modulators of NT levels. For instance, the probiotic Lactobacillus rhamnosus has been shown to affect GABA receptor expression, increasing GABAB1b mRNA in certain brain regions while decreasing its expression in others. This modulation has implications for stress-related disorders such as anxiety and depression.[93] Similarly, prebiotic chito-oligosaccharides, derived from chitin, have demonstrated strong inhibition of acetylcholinesterase, an enzyme responsible for the breakdown of acetylcholine. This inhibition suggests that prebiotics could be beneficial in preventing or treating cognitive disorders like AD by maintaining higher levels of acetylcholine in the brain.[94]
Drugs can also mimic the action of NTs, altering neurotransmission by interacting with specialized receptors and transporters. This mimicry can be harnessed therapeutically to modulate NT systems and address neurological and psychiatric conditions. However, drug abuse and addiction can disrupt these systems, leading to significant physiological and psychological dysfunctions. Therefore, understanding the precise mechanisms through which drugs interact with NT systems is crucial for developing effective therapeutic strategies.[95]
In summary, the modulation of NTs and their transporters presents a promising avenue for therapeutic intervention in neurological and neuropsychiatric disorders. By enhancing or inhibiting specific transporters and leveraging the influence of external agents such as drugs, probiotics, and prebiotics, it is possible to achieve more targeted and effective treatment outcomes. Continued research and development in this field has the potential to revolutionise the management of numerous brain illnesses, providing hope for a better quality of life for those affected.
Conclusion
NTs are chemical messengers that transport and amplify information throughout the nervous system, influencing feelings, ideas, perceptions, movements, learning, sleep cycles, behaviour, consciousness, excitement, blood flow, and respiration. Abnormalities in levels of neurotransmitters can cause serious disorders that affect both people and entire health systems. Variable amounts of NTs that include glutamate, GABA, dopamine, serotonin, norepinephrine, histamine, and acetylcholine are related with a range of illnesses, including autism spectrum disorders, schizophrenia, epilepsy, ALS, PD, HD, AD, drug addiction, depression, and sleep problems. Early detection and monitoring of neurotransmitter imbalances are crucial to prevent complications. Despite this, diagnosing brain disorders chemically remains complex, necessitating further research into the mechanisms of neurotransmitter action and strategies for modulating their levels. Overall, NTs are fundamental to understanding and treating numerous neurological and neurodegenerative conditions, and interdisciplinary research is key to developing effective treatments that can improve the quality of life for millions of patients globally.
Source of Funding
None.
Conflict of Interest
None.
References
- H Liu, X Liu. Nervous system. . Multi-System Imaging Spectrum associated with Neurologic Diseases 2023. [Google Scholar]
- TR Yoithapprabhunath, K Rachelsarahvinodhini, S Mohanapriya, JBS Raj, V Kalaiselvi, RM Nirmal. Neurotrophins (NTs) and Neurotrophin Receptors (NTRs) as emerging therapeutic paradigm in head and neck tumors - A mini review. J Indian Acad Oral Med Radiol 2020. [Google Scholar]
- SE Davis, AB Cirincione, AC Jimenez-Torres, J Zhu. The Impact of Neurotransmitters on the Neurobiology of Neurodegenerative Diseases. Int J Mol Sci 1920. [Google Scholar]
- A Gasmi, A Nasreen, A Menzel, G Benahmed, A Pivina, L Noor. Neurotransmitters Regulation and Food Intake: The Role of Dietary Sources in Neurotransmission. Molecules 2023. [Google Scholar]
- M Sánchez-Soto, A Bonifazi, NS Cai, MP Ellenberger, AH Newman, S Ferré. Evidence for noncanonical neurotransmitter activation: Norepinephrine as a dopamine D2-like receptor agonist. Mol Pharma 2016. [Google Scholar]
- X Xia, Y Wang, Y Qin, S Zhao, JC Zheng. Exosome: A novel neurotransmission modulator or non-canonical neurotransmitter?. Ageing Res Rev 2022. [Google Scholar]
- A Fozzato. Manipulating mitochondrial dynamics in the NTS prevents diet-induced deficits in brown fat morphology and glucose uptake. Life Sci 2022. [Google Scholar]
- JA Leite, A Orellana, PF Kinoshita, NP Mello, C Scavone, EM Kawamoto. Mechanisms of Neuroinflammation. Neuroinflammation and Neurotransmission Mechanisms Involved in Neuropsychiatric Disorders. 2017. [Google Scholar]
- B Si, E Song. Recent advances in the detection of neurotransmitters. Chemosensors 2018. [Google Scholar]
- Y Su, S Bian, M Sawan. Real-time in vivo detection techniques for neurotransmitters: A review. Analyst 2019. [Google Scholar]
- T Iman, R Akram, M S Javed, A Rasul, F Sajid, A Tehreem. The Role of Natural Antioxidants in Brain Disorders . Available Treatment Modules for Brain Disorders 2023. [Google Scholar]
- RA Webster. Drugs and Brain Function.. Neurotransmitters, Drugs and Brain Function 2001. [Google Scholar]
- O Bohlen. Neurotransmitters and Neuromodulators Handbook of Receptors and Biological Effects.. Neurodegenerative Diseases 2006. [Google Scholar]
- SD Niyonambaza, P Kumar, P Xing, J Mathault, P Koninck, E Boisselier. A Review of neurotransmitters sensing methods for neuro-engineering research. Appl Sci 2019. [Google Scholar]
- S Ito. GABA and glycine in the developing brain. J Physiol Sci 2016. [Google Scholar]
- KN Fedder, SL Sabo. On the role of glutamate in presynaptic development: Possible contributions of presynaptic NMDA receptors. Biomolecules 2015. [Google Scholar]
- J Lewerenz, P Maher. Chronic Glutamate Toxicity in Neurodegenerative Diseases-What is the Evidence? . Front Neurosci 2015. [Google Scholar]
- J Zou, YX Wang, FF Dou, HZ Lü, ZW Ma, PH Lu. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int 2010. [Google Scholar]
- R Doke, A Bhagwat, K Autade, G Lamkhade, A Wakchaure, T Naik. Anxiety and Depression: Ignored Neuropsychiatric Aspects of Parkinson’s. Dis Chem Bull 2023. [Google Scholar]
- R Mazzoli, E Pessione. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front Microb 2016. [Google Scholar]
- P Nuss. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatric Dis Treat 2015. [Google Scholar]
- BG Wallin, N Charkoudian. Sympathetic neural control of integrated cardiovascular function: Insights from measurement of human sympathetic nerve activity. . Muscle Nerve 2007. [Google Scholar]
- MS Hernandes, L Troncone. Glycine as a neurotransmitter in the forebrain: A short review. J Neu Trans 2009. [Google Scholar]
- SH Snyder, PM Kim. D-amino acids as putative neurotransmitters: Focus on D-serine. Neurochem Res 2000. [Google Scholar]
- M Nimgampalle, H Chakravarthy, S Sharma, S Shree, AR Bhat, JA Pradeepkiran. Neurotransmitter systems in the etiology of major neurological disorders: Emerging insights and therapeutic implications. Ageing Res Rev 2023. [Google Scholar]
- C Missale, SR Nash, SW Robinson, MG Caron, J Neurophysiol, A Ophthalmol. Dopamine receptors: from structure to function. Physiol Rev 2009. [Google Scholar]
- P Kumar, SN Abed, YA Bataineh, MS Salem. Neurotransmitters and Their Receptors- State of the Art. Front Pharm Neurotrans 2020. [Google Scholar]
- SM O’mahony, G Clarke, YE Borre, TG Dinan, JF Cryan. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 2015. [Google Scholar]
- SJ Sara. The locus coeruleus and noradrenergic modulation of cognition. Nature Rev Neurosci 2009. [Google Scholar]
- DL Wong, TC Tai, DC Wong-Faull, R Claycomb, EG Meloni, KM Myers. Epinephrine: A short- and long-term regulator of stress and development of illness: A potential new role for epinephrine in stress. Cell Mol Neurobiol 2012. [Google Scholar]
- H Qian, C Shu, L Xiao, G Wang. Histamine and histamine receptors: Roles in major depressive disorder. Front Psych 2022. [Google Scholar]
- JA Wilcox. Neurochemistry of Consciousness: Neurotransmitters in Mind. Am J Psych 2003. [Google Scholar]
- J P Aronson, H A Katnani, E N Eskandar. Neuromodulation for Obsessive-Compulsive Disorder. Neurosurgery Clinics of North America 2014. [Google Scholar]
- AT Pawar, C Jogdeo, A Upaganlawar, SB Chandrasekar, S Putta. Cholinergic Neurotransmission. Neurochemical Systems and Signaling: from Molecules to Networks. Prog Histochem Cytochem 2023. [Google Scholar]
- N Khatri, H Y Man. Synaptic activity and bioenergy homeostasis: Implications in brain trauma and neurodegenerative diseases. Frontiers in Neurology 2013. [Google Scholar]
- N Mrnjavac, WF Martin. GTP before ATP: The energy currency at the origin of genes. 2024. [Google Scholar]
- EW Godfrey, RC Schwarte. The role of nitric oxide signaling in the formation of the neuromuscular junction. J Neurocytol 2003. [Google Scholar]
- SH Snyder, CD Ferris. Novel neurotransmitters and their neuropsychiatric relevance. Am J Psych 2000. [Google Scholar]
- Y Dumont, D Jacques, JA St-Pierre, R Quirion. Neuropeptide y receptor types in the mammalian brain. Neuropeptide Y Drug Develop 1997. [Google Scholar]
- F Reichmann, P Holzer. Neuropeptide Y: A stressful review. Neuropeptides 2016. [Google Scholar]
- T Das, TK Das, A Khodarkovskaya, S Dash. Non-coding RNAs and their bioengineering applications for neurological diseases. Bioengineered 2021. [Google Scholar]
- F Mantile, P Franco, MP Stoppelli, GL Liguori. Biological role and clinical relevance of extracellular vesicles as key mediators of cell communication in cancer. Adv Biomem Lipid Self-Assem 2021. [Google Scholar]
- AM Janas, K Sapoń, T Janas, MHB Stowell, T Janas. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochimica et Biophysica Acta - Biomemb 2016. [Google Scholar]
- EA Makrygianni, GP Chrousos. Extracellular Vesicles and the Stress System. Neuroendocrinol 2023. [Google Scholar]
- C Agulhon, MY Sun, T Murphy, T Myers, K Lauderdale, TA Fiacco. Calcium signaling and gliotransmission in normal vs. Reactive astrocytes. Front Pharm 2012. [Google Scholar]
- M Pascual, F Ibáñez, C Guerri. . Exosomes as mediators of neuron-glia communication in neuroinflammation. Neural Regeneration Research 2020. [Google Scholar]
- IC Brás, TF Outeiro. Alpha-synuclein: Mechanisms of release and pathology progression in synucleinopathies. Cells 2021. [Google Scholar]
- S Singh, A Ahuja, S Pathak. Potential Role of Oxidative Stress in the Pathophysiology of Neurodegenerative Disorders. Comb Chem High Through Screen 2024. [Google Scholar]
- Y Hojo, S Higo, S Kawato, Y Hatanaka, Y Ooishi, G Murakami. Hippocampal synthesis of sex steroids and corticosteroids: Essential for modulation of synaptic plasticity. Front Endocrinol 2011. [Google Scholar]
- R Dalangin, A Kim, RE Campbell. The role of amino acids in neurotransmission and fluorescent tools for their detection. Int J Mol Sci 2020. [Google Scholar]
- N Ota, T Shi, J V. D- Sweedler. Aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 2012. [Google Scholar]
- P Maiti, J Manna, GL Dunbar, P Maiti. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Translat Neurodegen 2017. [Google Scholar]
- RN Rosenberg. . Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease 2015. [Google Scholar]
- GL Holmes. Role of glutamate and GABA in the pathophysiology of epilepsy. Ment Retard Dev Disab Res Rev 1995. [Google Scholar]
- J Huguenard. Neurotransmitter Supply and Demand in Epilepsy. Epilepsy Curr 2003. [Google Scholar]
- J Correale, M Marrodan, MC Ysrraelit. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines 2019. [Google Scholar]
- E Akyuz, BR Celik, FS Aslan, H Sahin, E Angelopoulou. Exploring the Role of Neurotransmitters in Multiple Sclerosis: An Expanded Review. ACS Chem Neurosci 2023. [Google Scholar]
- F Gao, X Yin, RA Edden, AC Evans, J Xu, G Cao. Altered hippocampal GABA and glutamate levels and uncoupling from functional connectivity in multiple sclerosis. Hippocampus 2018. [Google Scholar]
- I A Hoppenbrouwers, RQ Hintzen. Genetics of multiple sclerosis. Biochimica et Biophysica Acta - Mol Basis Dis 2011. [Google Scholar]
- JR Homberg, EJ Kyzar, M Nguyen, WH Norton, J Pittman, MK Poudel. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci Biobeh Rev 2016. [Google Scholar]
- T Gandhi, CC Lee. Neural Mechanisms Underlying Repetitive Behaviors in Rodent Models of Autism Spectrum Disorders. Front Cell Neurosci 2021. [Google Scholar]
- HY Kuo, FC Liu. Pathophysiological Studies of Monoaminergic Neurotransmission Systems in Valproic Acid-Induced Model of. Aut Spect Dis 2022. [Google Scholar]
- N Cortés, V Andrade, R Maccioni. Behavioral and Neuropsychiatric Disorders in Alzheimer’s Disease. J Alzheimer’s Dis 2018. [Google Scholar]
- RR Doke, PA Pansare, SR Sainani, VM Bhalchim, KR Rode, SR Desai. The Counteracting Performance of Phytoconstituents Against Neurodegeneration Involved in Parkinson’s Disease. J Sci Res 2021. [Google Scholar]
- S Desai, R Doke, P Pansare, S Sainani, V Bhalchim. Natural products: An emerging tool in parkinson’s disease therapeutics. IP Indian J Neurosci 2019. [Google Scholar]
- L Iovino, ME Tremblay, L Civiero. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J Pharm Sci 2020. [Google Scholar]
- FJ Arnold, AF Putka, U Raychaudhuri, S Hsu, RS Bedlack, CL Bennett. Revisiting Glutamate Excitotoxicity in Amyotrophic Lateral Sclerosis and Age-Related Neurodegeneration. Int J Mol Sci 2024. [Google Scholar]
- G Ambrosi, S Cerri, F Blandini. A further update on the role of excitotoxicity in the pathogenesis of Parkinson’s disease. J Neural Trans 2014. [Google Scholar]
- S Jamwal, P Kumar. Insight Into the Emerging Role of Striatal Neurotransmitters in the Pathophysiology of Parkinson’s Disease and Huntington’s Disease: A Review. Curr Neuropharm 2018. [Google Scholar]
- J Cha, AS Frey, SA Alsdorf, JA Kerner, CM Kosinski, L Mangiarini. Altered neurotransmitter receptor expression in transgenic mouse models of Huntington’s disease. Philos Trans Royal Soc Biol Sci 1386. [Google Scholar]
- V Bansal, I Chatterjee. Role of neurotransmitters in schizophrenia: a comprehensive study. Kuwait J Sci 2021. [Google Scholar]
- A Adell. Brain NMDA receptors in schizophrenia and depression. Biomolecules 2020. [Google Scholar]
- D Senkowski, J Gallinat. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psych 2015. [Google Scholar]
- M Laruelle. From dopaminergic to glutamatergic interventions. Curr Opin Pharmacol 2014. [Google Scholar]
- R Rajmohan, PH Reddy. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J Alzheimer’s Dis 2017. [Google Scholar]
- ZR Chen, JB Huang, SL Yang, FF Hong. Role of Cholinergic Signaling in Alzheimer’s. Dis Mol 2022. [Google Scholar]
- UE Lang, S Borgwardt. Molecular mechanisms of depression: Perspectives on new treatment strategies. Cell Physiol Biochem 2013. [Google Scholar]
- JD Tubbs, J Ding, L Baum, PC Sham. Systemic neuro-dysregulation in depression: Evidence from genome-wide association. Eur Neuropsychopharma 2020. [Google Scholar]
- L Gall, L Anakor, E Connolly, O Vijayakumar, UG Duddy, WJ Duguez. Molecular and cellular mechanisms affected in als. J Personalized Med 2020. [Google Scholar]
- PJ Whitehouse, JK Wamsley, MA Zarbin, DL Price, WW Tourtellotte, MJ Kuhar. Amyotrophic lateral sclerosis: Alterations in neurotransmitter receptors. Ann Neurol 1983. [Google Scholar]
- MA Bhat, S Dhaneshwar. Neurodegenerative Diseases: New Hopes and Perspectives. Curr Mol Med 2023. [Google Scholar]
- DM Krakauer, PJ Willems, A Hofman. Genetic epidemiology of amyotrophic lateral sclerosis. Clin Gen 2003. [Google Scholar]
- N Chauhan, S Soni, P Agrawal, Yps Balhara, U Jain. Recent advancement in nanosensors for neurotransmitters detection: Present and future perspective. Process Biochem 2020. [Google Scholar]
- S Banerjee, S Mccracken, Faruk Hossain, M Slaughter. Electrochemical Detection of Neurotransmitters. Biosensors 2020. [Google Scholar]
- BJ Sanghavi, OS Wolfbeis, T Hirsch, NS Swami. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 2015. [Google Scholar]
- A Azzouz, KY Goud, N Raza, E Ballesteros, SE Lee, J Hong. Nanomaterial-based electrochemical sensors for the detection of neurochemicals in biological matrices. TrAC - Trend Anal Chem 2019. [Google Scholar]
- M Niello, R Gradisch, C J Loland, T Stockner, H H Sitte. Allosteric Modulation of Neurotransmitter Transporters as a Therapeutic Strategy. Trends in Pharmacological Sciences 2020. [Google Scholar]
- S Bhat, A El-Kasaby, M Freissmuth, S Sucic. Functional and Biochemical Consequences of Disease Variants in Neurotransmitter Transporters: A Special Emphasis on Folding and Trafficking Deficits. Pharmacol Therap 2021. [Google Scholar]
- A Armada-Moreira, JI Gomes, CC Pina, OK Savchak, J Gonçalves-Ribeiro, N Rei. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front Cell Neurosci 2020. [Google Scholar]
- E Pajarillo, A Rizor, J Lee, M Aschner, E Lee. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019. [Google Scholar]
- MH Cheng, I Bahar. Monoamine transporters: structure, intrinsic dynamics and allosteric regulation. Nat Struct Mol Biol 2019. [Google Scholar]
- F Alasmari. Caffeine induces neurobehavioral effects through modulating neurotransmitters. Saudi Pharm J 2020. [Google Scholar]
- A Sarkar, SM Lehto, S Harty, TG Dina, JF Cryan, P Burnet. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends in Neurosci 2016. [Google Scholar]
- JA Bravo, P Forsythe, MV Chew, E Escaravage, HM Savignac, TG Dinan. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceed Nat Acad Sci USA 2011. [Google Scholar]
- . The defining features of drug intoxication and addiction can be traced to disruptions in neuron-to neuron signaling. NIDA Notes. 2017. [Google Scholar]
- Introduction
- Neurotransmitters
- Neurotransmitter Disorders of the CNS
- Epilepsy
- Multiple sclerosis
- Autism
- Alzheimer's disease
- Parkinsons disease
- Huntington's disease
- Schizophrenia
- Depression
- Amyotrophic lateral sclerosis
- Neurotransmitters Detection
- Modulation of Neurotransmitters and Neurotransmitter Transporters as a Therapeutic Strategy
- Conclusion
- Source of Funding
- Conflict of Interest
