- Received August 03, 2024
- Accepted September 23, 2024
- Publication November 26, 2024
- Visibility 3 Views
- Downloads 0 Downloads
- DOI 10.18231/j.ijcaap.2024.042
-
CrossMark
- Citation
Medical writing in pharmacovigilance: An overview
- Author Details:
-
Abhijit Anil Trailokya *
-
Sunil Chaudhary
Introduction
“The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible drug-related problems is called as Pharmacovigilance”. [WHO, 2002]
Pharmacovigilance medical writing encompasses all stages of drug development, starting from the initial clinical trials to the essential report submissions required for market authorization and continuing into the post-marketing phase. This comprehensive involvement ensures that drug safety information is meticulously documented, communicated, and updated throughout the lifecycle of the drug. By addressing each phase, medical writing supports the accurate reporting of adverse events, facilitates regulatory compliance, and contributes to ongoing drug safety evaluations, ultimately safeguarding public health and maintaining the integrity of the pharmaceutical industry.[1]
Pharmacovigilance medical writing has a significant impact on both the clinical development and post-marketing phases of drug development. It plays a vital role in documenting and analyzing safety data, helping to assess and manage risks throughout the drug's lifecycle. Additionally, it is crucial in preparing required submission documents, such as safety reports and risk management plans, which are necessary for regulatory authorities to evaluate the drug's safety profile before granting marketing authorization or approval.
The marketing authorization holder is responsible for continuously monitoring the safety of its medicinal products for human use, for informing the authorities of any changes that might have an impact on the marketing authorization, and for ensuring that the product information is kept up-to-date.[2]
Medical Writing in Pharmacovigilance [3]
Medical writing involved in preparing various pharmacovigilance documents that needs to be submitted to regulatory authorities throughout the life cycle of any given medicinal product, starting with safety documentation required during clinical development, followed by safety documents required to support applications for marketing authorization, including RMPs, and finally those documents, such as the PSUR, that are required throughout the product’s post-marketing life. (Refer[Figure 1]).
Pharmacovigilance medical writing impacts on the clinical development and post-marketing phases, as well as making a significant contribution to the mandated submission documents required before the regulating authorities can grant marketing authorization/approval.
Medical writing plays a pivotal role in pharmacovigilance by crafting clear, precise, and regulatory-compliant documents that communicate safety information effectively.
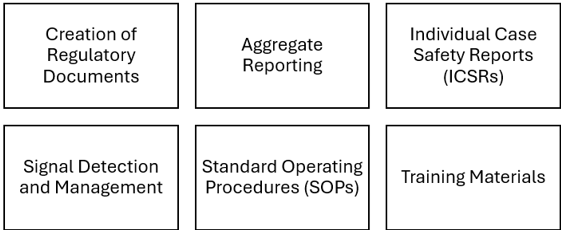
Creation of Regulatory Documents: Medical writers are responsible for preparing a variety of regulatory documents required for the approval and ongoing assessment of pharmaceuticals.
These include:
Risk Management Plans (RMPs): These documents outline the identified and potential risks of a drug and describe measures to minimize these risks.
Periodic Safety Update Reports (PSURs): PSURs are critical for providing a comprehensive analysis of a drug's risk-benefit profile at regular intervals.
Development Safety Update Reports (DSURs): DSURs focus on the safety data collected during the clinical trial phase.
These documents must meet stringent regulatory requirements and present data in a clear and scientifically accurate manner.
Aggregate Reporting: Medical writers compile aggregate reports that integrate safety data from various sources over a specified period. These reports, such as PSURs and DSURs, are essential for ongoing risk assessment. They involve: 1. Data Analysis and Interpretation: Analyzing adverse event data to identify trends and new safety signals. 2.Literature Reviews: Reviewing published literature for new safety information related to the drug.3. Benefit-Risk Evaluation: Assessing the overall benefit-risk profile of the drug based on the accumulated safety data.
Individual Case Safety Reports (ICSRs): ICSRs document individual adverse events reported by healthcare professionals or patients. Medical writers play a role in
Narrative Writing: Crafting clear and concise narratives that describe the adverse event, its outcome, and the suspected drug's involvement.
Quality Control: Ensuring that the ICSRs are complete, accurate, and meet regulatory standards.
Signal Detection and Management: Medical writers support pharmacovigilance teams in signal detection and management by: 1. Documentation: Preparing documents that describe the detected signals, the methods used for detection, and the subsequent actions taken. Communication: Creating reports and summaries that communicate new safety signals to regulatory authorities and other stakeholders.
Standard Operating Procedures (SOPs): Medical writers contribute to the development and maintenance of SOPs that outline pharmacovigilance processes. These documents ensure consistency and compliance with regulatory requirements across pharmacovigilance activities.
Training Materials: To keep pharmacovigilance professionals informed about the latest guidelines and procedures, medical writers develop training materials, including:
Training Manuals: Detailed guides on pharmacovigilance practices.
Presentations and Workshops: Educational resources for in-house training sessions.
Important Documents in Pharmacovigilance
Aggregate safety reports
Aggregate safety reports play a crucial role in assessing the safety of a medicinal product throughout its entire life cycle. While individual case safety reports offer insights into adverse events linked to a specific medication in a single patient, it is essential to analyze cumulative safety data collected globally. This broader analysis helps in understanding the overall safety profile and keeping track of the benefit-risk balance of the product.[4]
PBRER = periodic benefit-risk evaluation report
The goal of the Periodic Benefit-Risk Evaluation Report (PBRER) is to assess the balance between the benefits and risks of a medicinal product, considering new information in relation to cumulative data. It is submitted periodically by marketing authorization holders to regulatory authorities as a requirement during the post-authorization phase.[5]
Risk management plans (RMPs)
Risk Management Plans (RMPs) provide details on a medicine's safety profile, strategies for preventing or minimizing risks in patients, plans for studies and other activities to further understand the medicine's safety and efficacy, and methods for assessing the effectiveness of risk minimization measures.[6]
Development safety update report (DSUR)
The Development Safety Update Report (DSUR) provides safety information for drugs in clinical development. It serves to offer an annual, comprehensive review and evaluation of relevant safety data collected during the reporting period for an investigational drug, regardless of its market availability.
Periodic safety update reports (PSURs)
Periodic Safety Update Reports (PSURs) are pharmacovigilance documents designed to assess the risk-benefit profile of a medicinal product at specific intervals following its approval. The purpose of the PSUR is to offer a thorough and critical analysis of this risk-benefit balance, factoring in newly available safety data alongside existing information on the risks and benefits of the product.000[7]
Periodic adverse drug experience reports (PADERs)
Periodic Adverse Drug Experience Reports (PADERs) are cumulative safety reports required by the U.S. Food and Drug Administration (FDA) for products that have been approved for marketing in the United States. The submission of a PADER begins once a product has received marketing authorization. These reports are submitted on a quarterly and annual basis, with deadlines of 30 and 60 days after the data lock point, respectively. PADERs primarily present case reports involving serious, unlisted events (15-day alert reports), typically in narrative or tabular format. [4]
Important documents for pharmacovigilance at different stages of drug development[Table 1] [8]
|
Documents |
Purpose of documents |
|
1. Development Safety Update Report (DSUR): mainly for European Union (EU) |
Documents serve as a means for monitoring the safety of subjects involved in clinical studies by the sponsoring company, regulatory authorities, ethics committees, and institutional review boards. |
|
Common Technical Documentation CTD modules (i.e. CTD Modules 2.5.5 and 2.7.4) |
These documents contain comprehensive analyses of all safety data gathered during the clinical development of the medicinal product. They serve as the foundation for the product's labeling and the complete safety information provided to prescribers and healthcare professionals once the product has been granted marketing authorization (i.e., licensed for use). |
|
Integrated Summary of Safety (ISS) |
|
|
Risk Management Plan (RMP) |
This document outlines the safety information that remains to be established for the medicinal product and details the steps the company will take to address these gaps in the product's safety profile. Additionally, the RMP details the steps the company will take to mitigate the known safety risks of the product and how the effectiveness of these efforts will be assessed and monitored. |
|
Benefit-Risk Evaluation Report |
It evaluates the benefits of using the medicinal product against the risks for a specific patient population and treatment indication, to determine if the product has a favorable benefit-risk profile (i.e., if the benefits outweigh or justify the potential risks). |
|
Periodic safety update reports (PSURs) |
Periodic Safety Update Reports (PSUR), Periodic Adverse Drug Experience Reports (PADER), and related documents such as the PSUR Addendum and Summary Bridging Report (SBR) must be submitted to regulatory agencies at specified intervals after a product is authorized for marketing. These reports play a key role in monitoring the product's safety and allow for the quick identification and management of any emerging risks or changes in the safety profile. |
|
Periodic Adverse Experience Reports [PADERs] for the US region |
|
|
PSUR Addendums |
|
|
Summary Bridging Reports (SBR) |
|
Career as a Pharmacovigilance Writer[9]
Pharmacovigilance is an integral part of drug development & clinical research and is growing globally. Many pharmacovigilance centers are working for drug safety monitoring in this global boom, however, pharmacovigilance systems face major human resource challenges and there is a perennial need for skilled professionals. New drugs take a long time to market and there is a steep regulatory course in which safety forms a vital component for achieving the final goal of marketing authorization. Pharmacovigilance writers do not only work with pharmaceutical companies but can find employment with allied industry sectors like contract research organizations, BPO/KPOs, site management organizations, regulatory companies or consultants, government regulatory agencies, etc.
Many leading pharmaceutical companies today are looking for drug safety professionals to be a part of their industry and help in making a contribution with their efficient skills to this industry. The Professional Diploma in Pharmacovigilance and Pharmacoepidemiology is one of the courses that will provide the basis in pharmacovigilance principles and operations.
Conclusion
In summary, medical writing plays a crucial role in pharmacovigilance, acting as the foundation for clear and accurate communication of drug safety information. The accuracy and clarity of documents such as PSURs, PADERs, and RMPs are crucial for ensuring that regulatory authorities, healthcare professionals, and patients have access to up-to-date and reliable information about drug risks and benefits. Medical writers must navigate complex regulatory requirements and synthesize extensive clinical data to produce documents that uphold the highest standards of quality and compliance. As pharmacovigilance continues to evolve with advancements in drug development and regulatory expectations, the role of medical writing remains essential in safeguarding public health and maintaining the integrity of the pharmaceutical industry.
Source of Funding
None.
Conflict of Interest
None.
References
- . Pharmacovigilance Medical Writing: A new career trend. 2024. [Google Scholar]
- M Mammì, R Citraro, G Torcasio, G Cusato, C Palleria, ED Di Paola. Pharmacovigilance in pharmaceutical companies: An overview. J Pharm Pharmacother 2013. [Google Scholar]
- Von Bruchhausen. Pharmacovigilance medical writing: An evolving profession. Medical Writing 2015. [Google Scholar]
- TN Kulkarni, NG Kulkarni. Authoring a periodic adverse drug experience report here's what you need to know! Perspect Clin Res. Perspect Clin Res 2019. [Google Scholar]
- S Sharan. IS0P 2021 Annual Meeting – Post Conference Course III PBRER Making & Assessment. 2021. [Google Scholar]
- . Risk management plans. . [Google Scholar]
- . Periodic safety update reports (PSURs). . [Google Scholar]
- JO Lindsay. . Pharmacovigilance Medical Writing: A Good Practice Guide 2012. [Google Scholar]
- . Pharmacovigilance: A Career Opportunity You Must Not Miss. 2024. [Google Scholar]
- Introduction
- Medical Writing in Pharmacovigilance [3]
- Important Documents in Pharmacovigilance
- Aggregate safety reports
- PBRER = periodic benefit-risk evaluation report
- Risk management plans (RMPs)
- Development safety update report (DSUR)
- Periodic safety update reports (PSURs)
- Periodic adverse drug experience reports (PADERs)
- Career as a Pharmacovigilance Writer[9]
- Conclusion
- Source of Funding
- Conflict of Interest
